Geological observations of Mars indicate a dense early atmosphere ranging from 0.25 to 4 bar of carbon dioxide. But 3.5 billion years ago, the Martian atmosphere thinned rapidly to approximately 0.054 bar, suggesting a substantial loss of atmospheric carbon dioxide, either to space or the lithosphere. The mechanism by which Mars lost its carbon dioxide remains poorly understood. For MIT geologists Joshua Murray and Oliver Jagoutz, the answer may lie in the planet’s clay-covered crust. The researchers used their knowledge of interactions between rocks and gases on Earth and applied that to how similar processes could play out on Mars. They found that, given how much clay is estimated to cover the Martian surface, the planet’s clay could hold up to 1.7 bar of carbon dioxide, which would be equivalent to around 80% of the planet’s initial, early atmosphere. It’s possible that this sequestered Martian carbon could one day be recovered and converted into propellant to fuel future missions between Mars and Earth, the researchers propose.

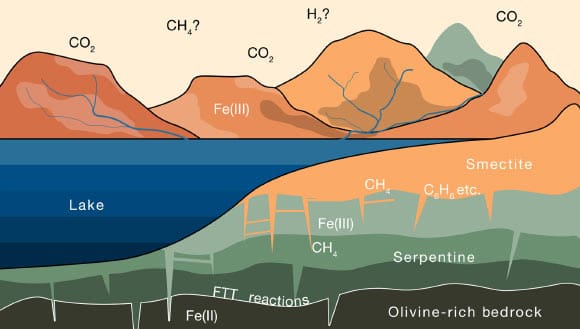
This schematic illustrates the progressive alteration of iron-rich rocks on Mars as the rocks interact with water containing carbon dioxide from the atmosphere. Image credit: Joshua Murray & Oliver Jagoutz, doi: 10.1126/sciadv.adm8443.
“Based on our findings on Earth, we show that similar processes likely operated on Mars, and that copious amounts of atmospheric carbon dioxide could have transformed to methane and been sequestered in clays,” Professor Jagoutz said.
“This methane could still be present and maybe even used as an energy source on Mars in the future.”
Professor Jagoutz and Murray seek to identify the geologic processes and interactions that drive the evolution of Earth’s lithosphere — the hard and brittle outer layer that includes the crust and upper mantle, where tectonic plates lie.
In 2023, they focused on a type of surface clay mineral called smectite, which is known to be a highly effective trap for carbon.
Within a single grain of smectite are a multitude of folds, within which carbon can sit undisturbed for billions of years.
They showed that smectite on Earth was likely a product of tectonic activity, and that, once exposed at the surface, the clay minerals acted to draw down and store enough carbon dioxide from the atmosphere to cool the planet over millions of years.
Soon after they reported their results, Professor Jagoutz happened to look at a map of the surface of Mars and realized that much of that planet’s surface was covered in the same smectite clays.
Could the clays have had a similar carbon-trapping effect on Mars, and if so, how much carbon could the clays hold?
Unlike on Earth, where smectite is a consequence of continental plates shifting and uplifting to bring rocks from the mantle to the surface, there is no such tectonic activity on Mars.
The scientists looked for ways in which the clays could have formed on Mars, based on what they know of the planet’s history and composition.
For instance, some remote measurements of Mars’ surface suggest that at least part of the planet’s crust contains ultramafic igneous rocks, similar to those that produce smectites through weathering on Earth.
Other observations reveal geologic patterns similar to terrestrial rivers and tributaries, where water could have flowed and reacted with the underlying rock.
The authors wondered whether water could have reacted with Mars’ deep ultramafic rocks in a way that would produce the clays that cover the surface today.
They developed a simple model of rock chemistry, based on what is known of how igneous rocks interact with their environment on Earth.
They applied this model to Mars, where scientists believe the crust is mostly made up of igneous rock that is rich in the mineral olivine.
The team used the model to estimate the changes that olivine-rich rock might undergo, assuming that water existed on the surface for at least a billion years, and the atmosphere was thick with carbon dioxide.
“At this time in Mars’ history, we think carbon dioxide is everywhere, in every nook and cranny, and water percolating through the rocks is full of carbon dioxide too,” Murray said.
Over about a billion years, water trickling through the crust would have slowly reacted with olivine — a mineral that is rich in a reduced form of iron.
Oxygen molecules in water would have bound to the iron, releasing hydrogen as a result and forming the red oxidized iron which gives the planet its iconic color.
This free hydrogen would then have combined with carbon dioxide in the water, to form methane.
As this reaction progressed over time, olivine would have slowly transformed into another type of iron-rich rock known as serpentine, which then continued to react with water to form smectite.
“These smectite clays have so much capacity to store carbon,” Murray said.
“So then we used existing knowledge of how these minerals are stored in clays on Earth, and extrapolate to say, if the Martian surface has this much clay in it, how much methane can you store in those clays?”
The researchers found that if Mars is covered in a layer of smectite that is 1,100 m deep, this amount of clay could store a huge amount of methane, equivalent to most of the carbon dioxide in the atmosphere that is thought to have disappeared since the planet dried up.
“We find that estimates of global clay volumes on Mars are consistent with a significant fraction of Mars’ initial carbon dioxide being sequestered as organic compounds within the clay-rich crust,” Murray said.
“In some ways, Mars’ missing atmosphere could be hiding in plain sight.”
The results appear in the journal Science Advances.
_____
Joshua Murray & Oliver Jagoutz. 2024. Olivine alteration and the loss of Mars’ early atmospheric carbon. Science Advances 10 (39); doi: 10.1126/sciadv.adm8443
This article is based on a press-release provided by MIT.